Time Medical International Ventures (India) Private Limited (Wholly Owned Subsidiary of Fischer Medical Ventures Limited), Receives India’s First CDSCO License for Manufacturing and Distribution of MRI Systems
Time Medical International Ventures (India) Limited, India’s first indigenous MRI manufacturer has become the first MRI company in India to receive the CDSCO license for manufacturing and distribution of MRI systems. This achievement supports the Make in India initiative with FDA and CE-approved models like EMMA, PICA, MICA, and QUIN. These MRI systems meet global safety and quality standards, enhancing India's healthcare capabilities.
Vishakapatnam, Andhra Pradesh – Time Medical International Ventures (India) Private Limited (Wholly Owned Subsidiary of Fischer Medical Ventures Limited), a Make in India company, is proud to announce that it has become the first indigenous MRI manufacturer in India to receive the official License to Manufacture for Sale or Distribution of Magnetic Resonance Diagnostic Devices (MRDD) from the Central Drugs Standard Control Organization (CDSCO). This achievement marks a significant milestone in the Make in India initiative, reinforcing the company’s commitment to developing high-quality, locally manufactured medical devices that meet international standards.
With this new license, Time Medical will manufacture and distribute the following MRI systems, some of which are FDA and CE approved as well, ensuring compliance with global safety, quality, and regulatory standards:
• EMMA – 1.5T MRI Scanner
• PICA – 0.35T MRI Scanner
• MICA – 1.5T MRI Scanner
• QUIN – 1.5T MRI Scanner
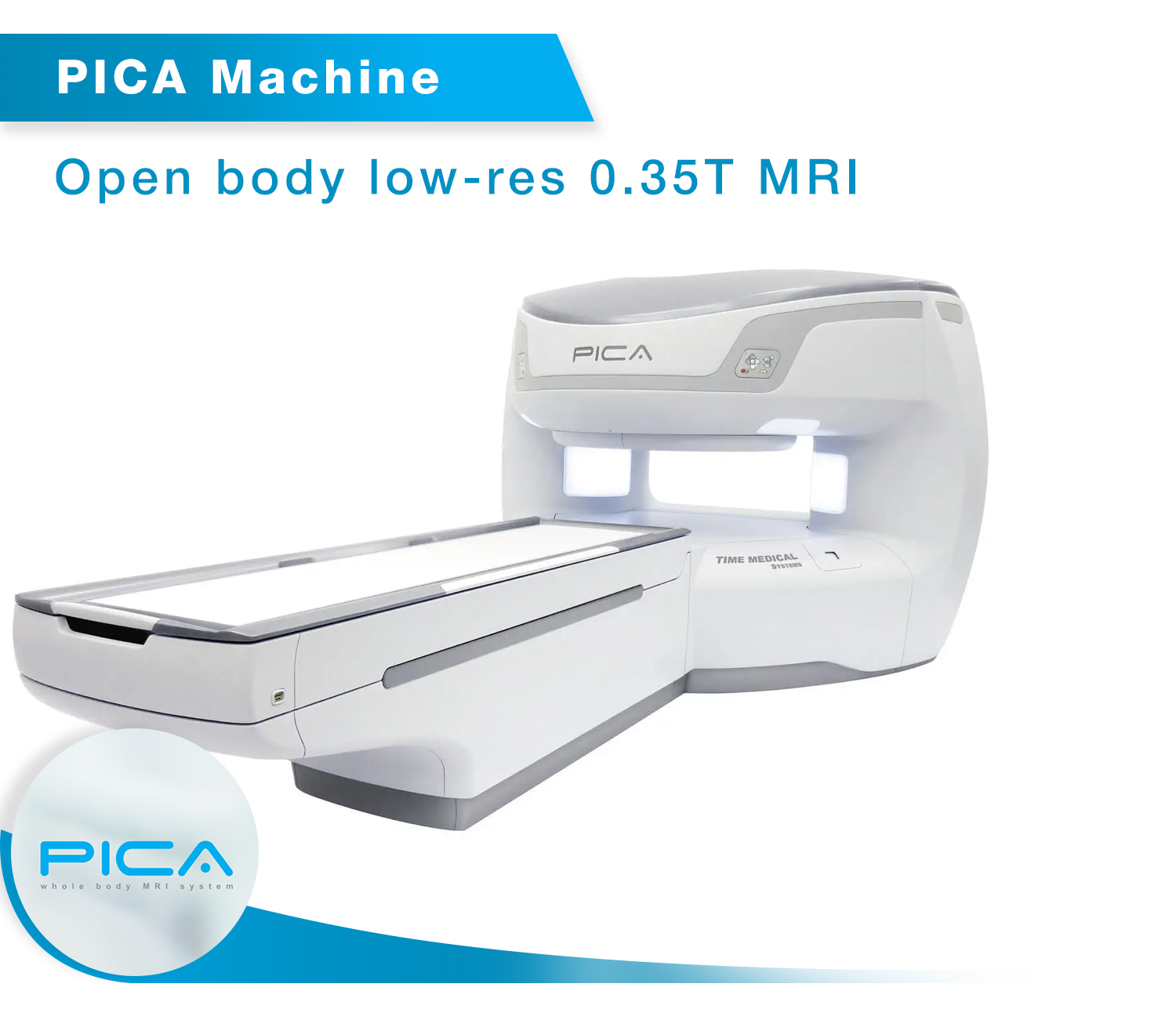

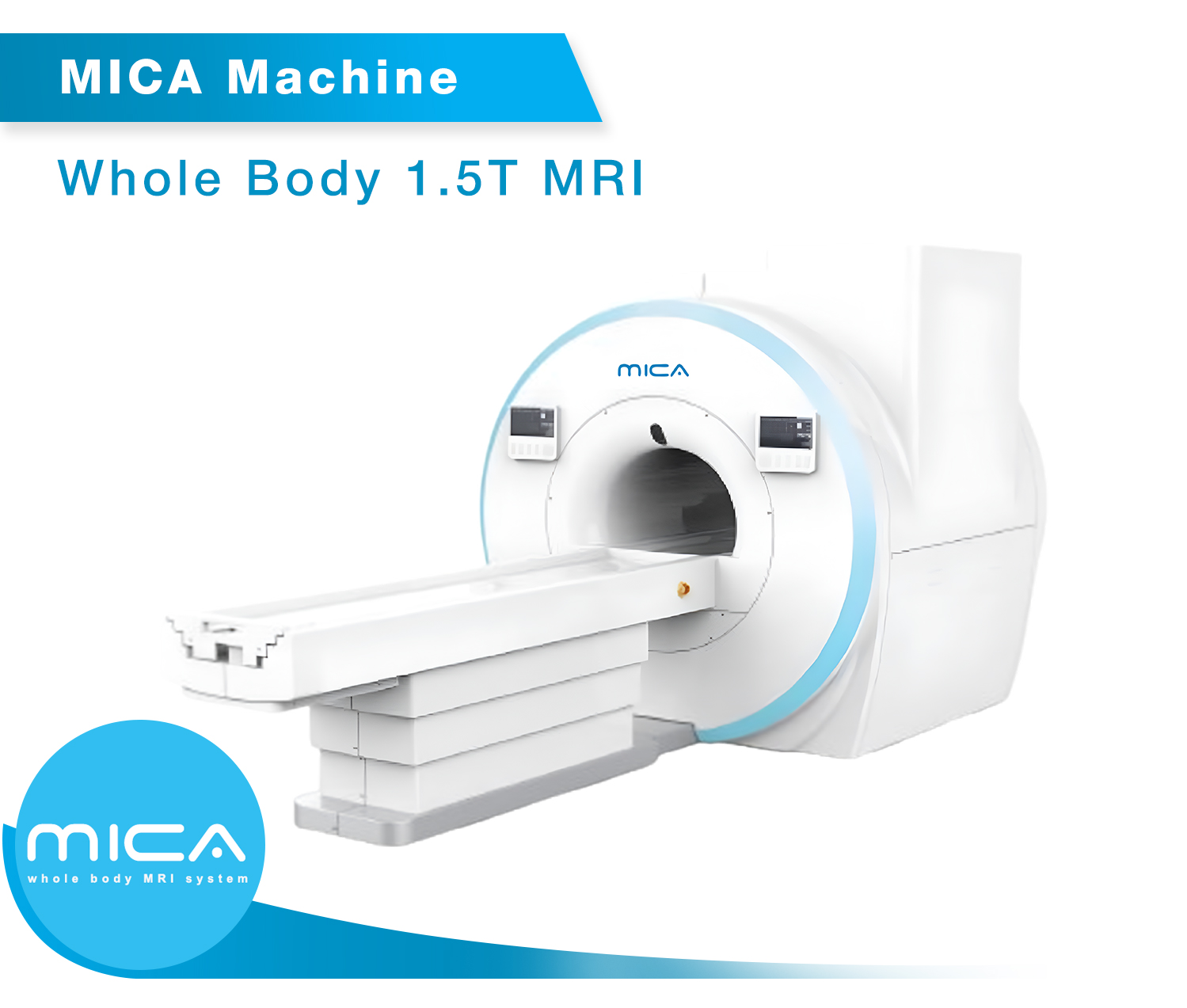
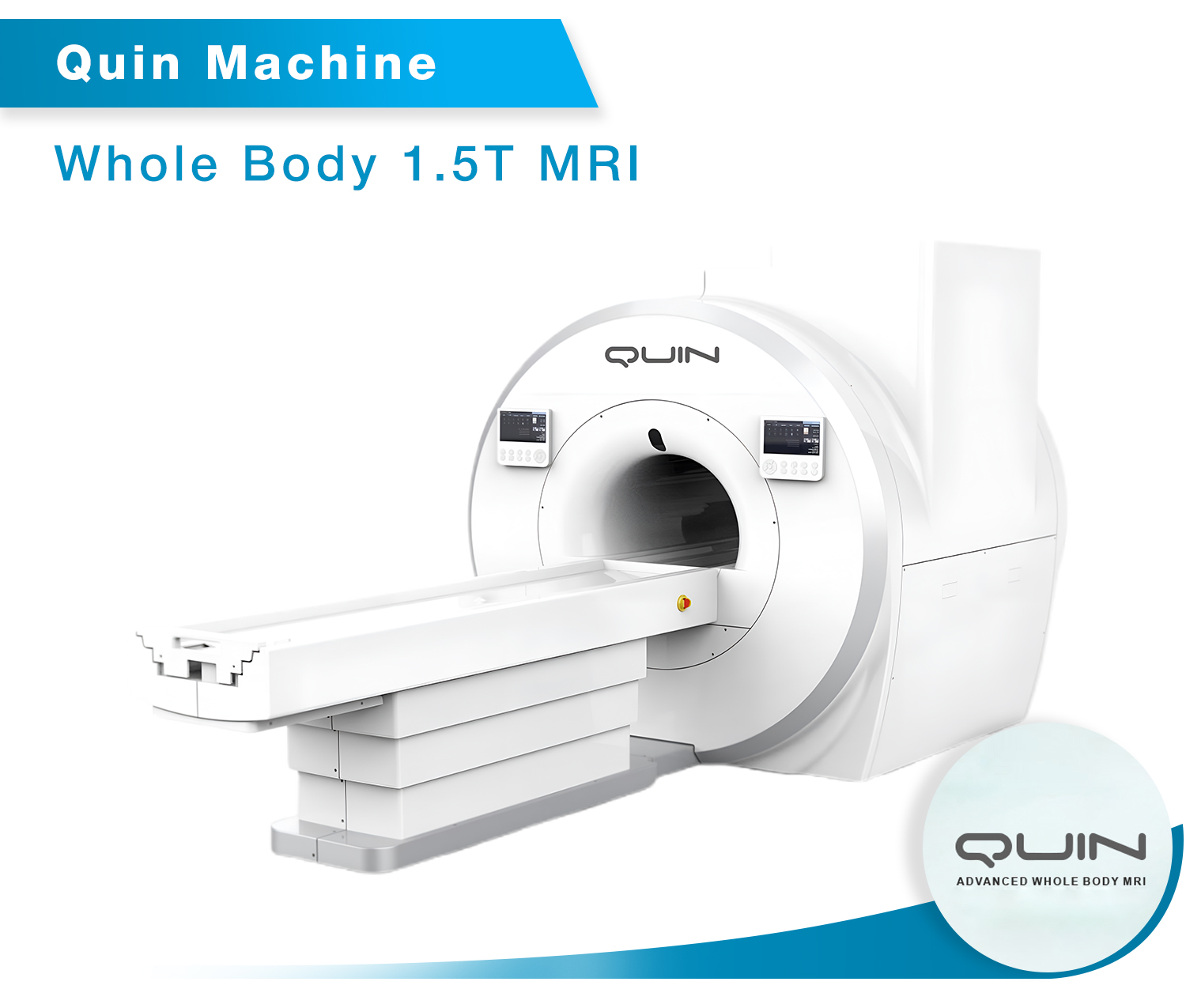
As a proud Make in India initiative, Time Medical is the first company in India to manufacture MRI systems that meet international safety and performance standards. The EMMA and PICA MRI models, which are FDA and CE approved, have undergone stringent clinical evaluations and are compliant with the highest international regulatory standards. This milestone reinforces India’s capability to produce world-class medical devices, contributing to the country’s growing healthcare infrastructure.
“We are thrilled to be the first Make in India company to receive the CDSCO license for the manufacturing and distribution of MRI systems,” said the Management Team of Fischer Medical Ventures. “This achievement demonstrates the strength and potential of India’s medical device manufacturing sector. With our EMMA, PICA, MICA, and QUIN MRI systems, we are committed to providing hospitals and diagnostic centers with cutting-edge, affordable diagnostic imaging solutions.
“The EMMA, MICA, and QUIN MRI systems are 1.5T MRI scanners, offering exceptional imaging resolution and precision for a wide range of clinical applications, while the PICA model is a 0.35T MRI scanner, tailored for specific diagnostic needs. These models are available for distribution across India, helping to address the growing demand for reliable and advanced medical imaging solutions.”
Fischer Medical Ventures is proud to contribute to the Make in India initiative by providing high quality, locally manufactured MRI systems that can enhance diagnostic capabilities and improve healthcare delivery across the country.



